Work Package 1
Efficacy assessment of Rifampicin and Albendazole to treat Onchocerciasis patients
There is a global quest to get a drug or combination of drugs, that can be delivered in 10 days or less, with adult worm-killing / macrofilaricidal effects in the treatment of onchocerciasis patients. Hence, the need to undertake a clinical trial that aims to shorten the treatment period and assess the safety and adult filarial worm-killing efficacy of the drug combination of rifampicin and albendazole in onchocerciasis-infected individuals. It is expected at the end of the trial that, the combination of these two drugs administered continuously for 7 and 14 days, respectively, will be successful in treating onchocerciasis patients. In preclinical studies, the combination of high-dose rifampicin with albendazole for seven days resulted in an increased macrofilaricidal effect. Wolbachia, which is an endosymbiotic bacteria of the onchocerciasis filarial worm, was depleted to above a 90% threshold in living adult female worms, predictive of a permanent adult worm-killing activity. Therefore, in this trial, a comparison among enrolled study participants in the following arms will be undertaken:
- Rifampicin plus Albendazole for 7 days
- Rifampicin plus Albendazole for 14 days
- Albendazole only for 14 days
- Control/placebo / no treatment.
Enrolled study participants in the treatment arms will be treated with a dosage of Rifampicin 35mg/kg/d and Albendazole 400 mg/d.
One hundred and twenty (120) onchocerciasis study participants were recruited and enrolled on this work package during the first quarter of 2021. Enrolled study participants are currently in the follow-up phase. The trial is registered with the Pan African Clinical Trial Registry. For more details on the trial protocol, see PACTR202009704006025. The pictures below show some of the procedures undertaken by the research team while carrying out work package 1.

Meeting with the opinion leaders of a community for their consent and approval before survey is scheduled

Constructing tent on the field to ensure participant privacy and confidentiality during palpation for onchocercal nodules and skin snipping

Explaining research procedures to potential study participants. This helps these individuals to make informed decision as to whether or not they will want to take part in the study.
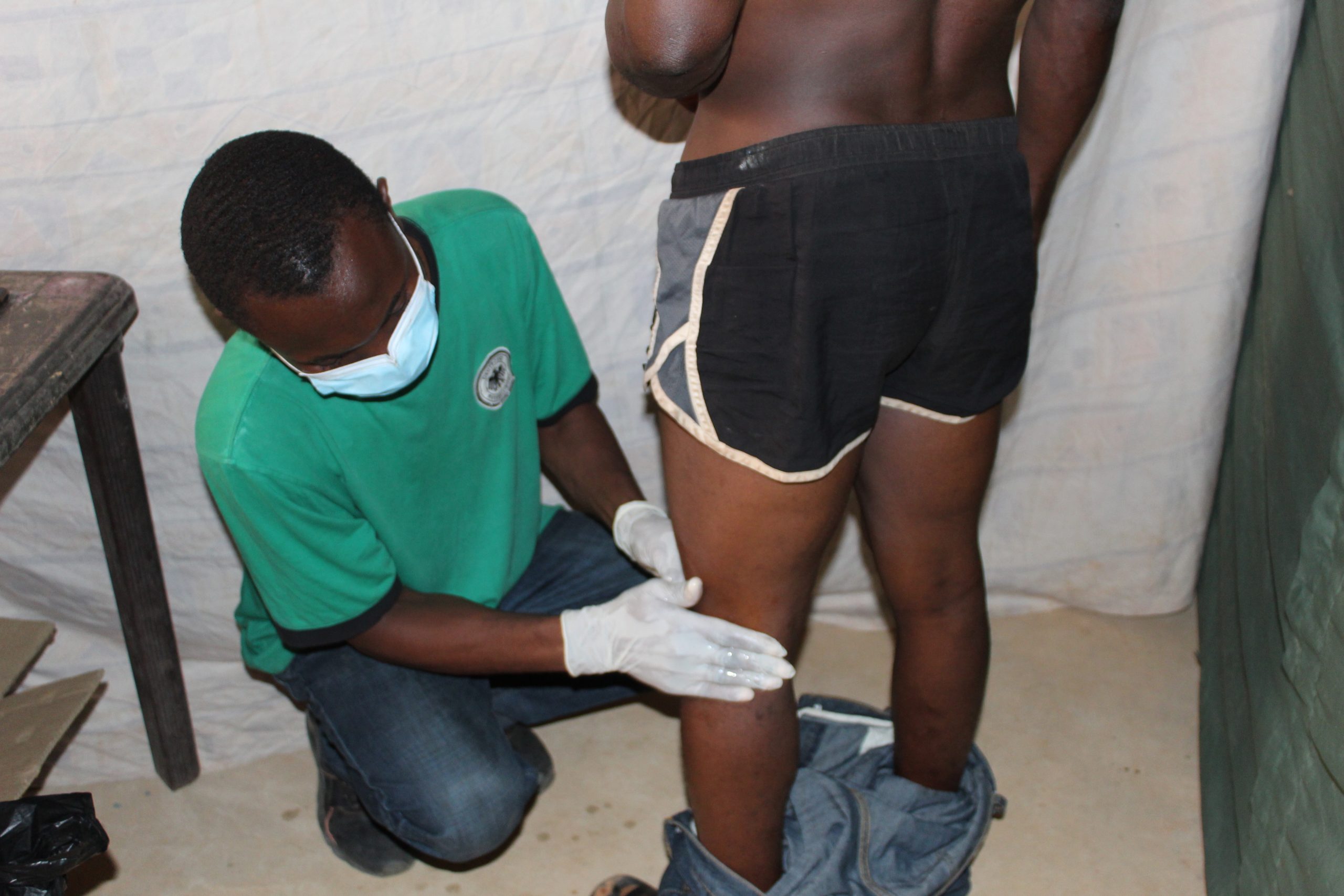
A potential study participantt undergoing palpation procedure for onchocercal nodules and skin examination for other onchocercal dermatitis. This is done after signing the consent form or thumbprinting it to confirm voluntary participation of the study.
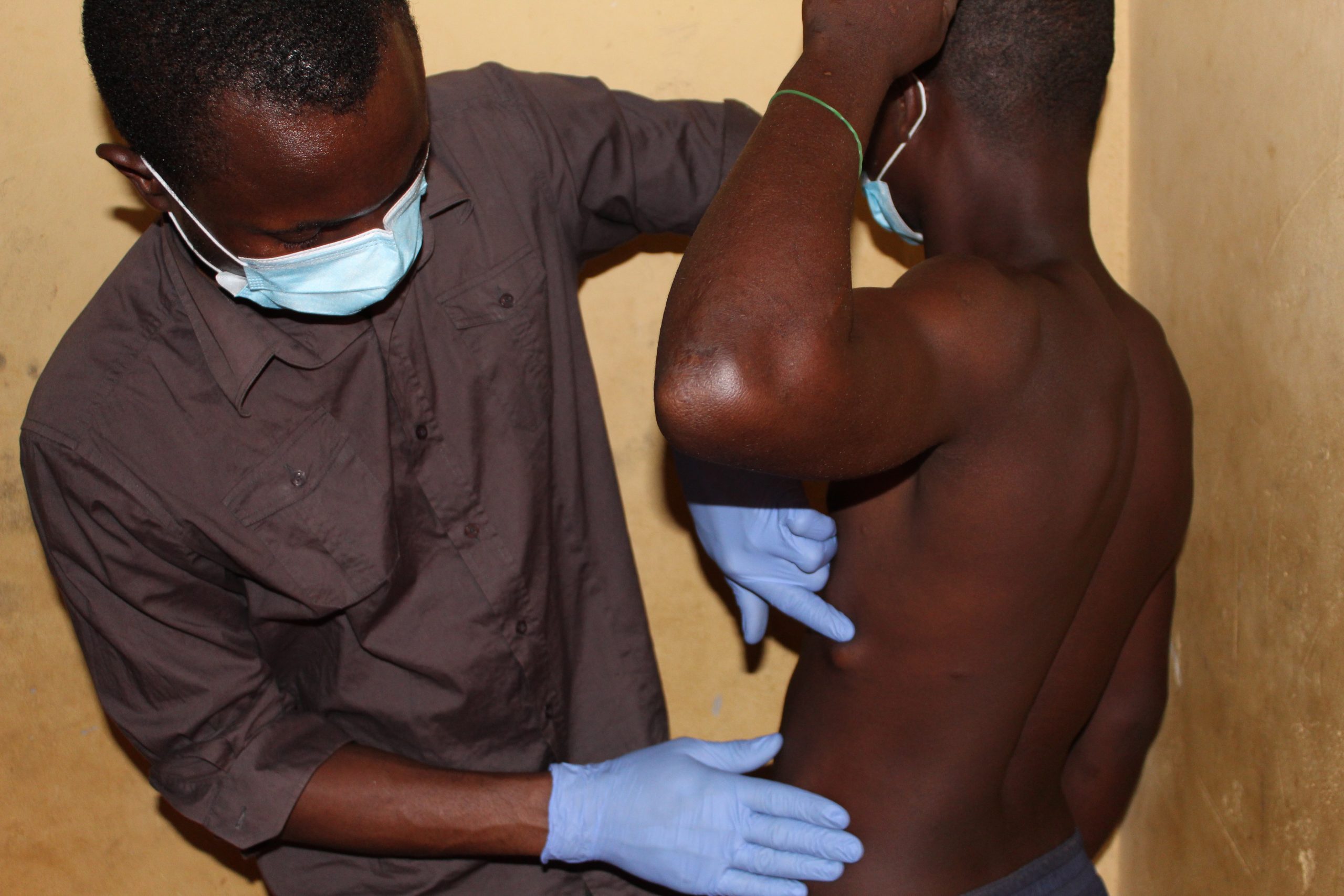
Study participant with onchocercal nodule on the lateral part of the left ribs
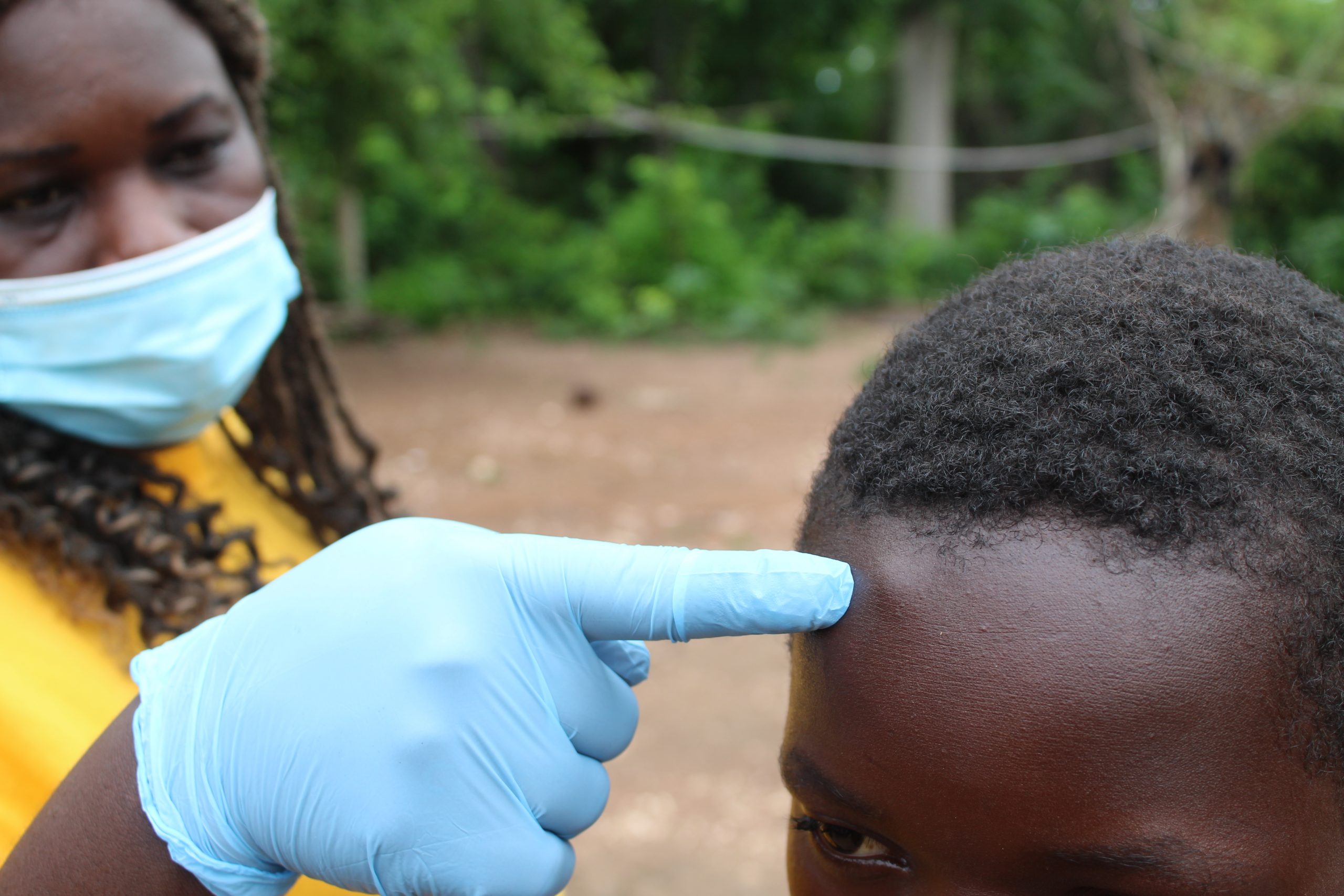
Study participant with onchocercal nodule on the forehead
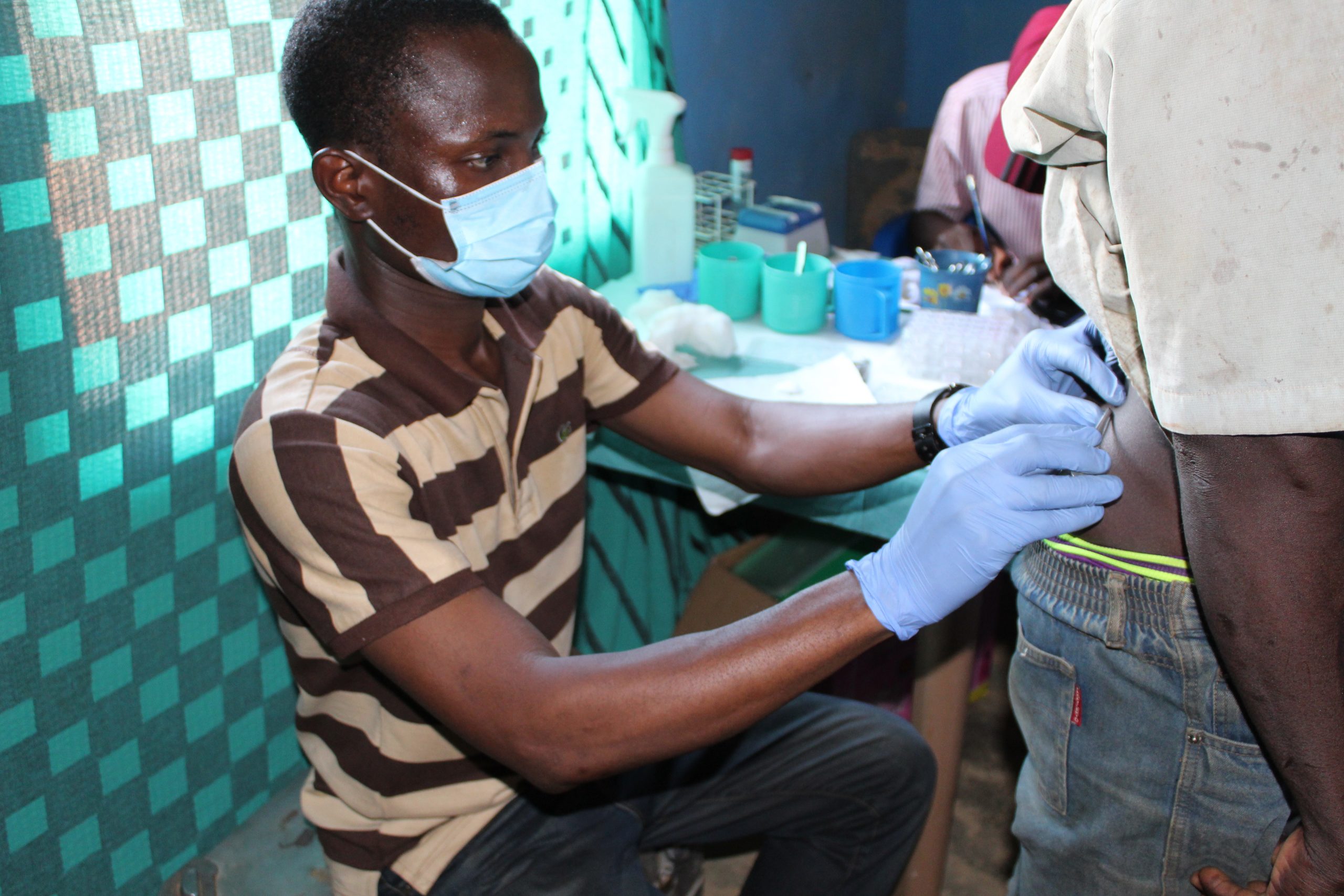
Snipping a nodule positive person on the iliac crest to be examined microscopically for the baby worms (microfilariae) of Onchocerca volvulus. This process is carried out in an enclosed area to ensure participant privacy and confidentiality.

Microscopic examination of the skin biopsies for the baby worms (microfilariae) of Onchocerca volvulus 24 hours after the skin snipping
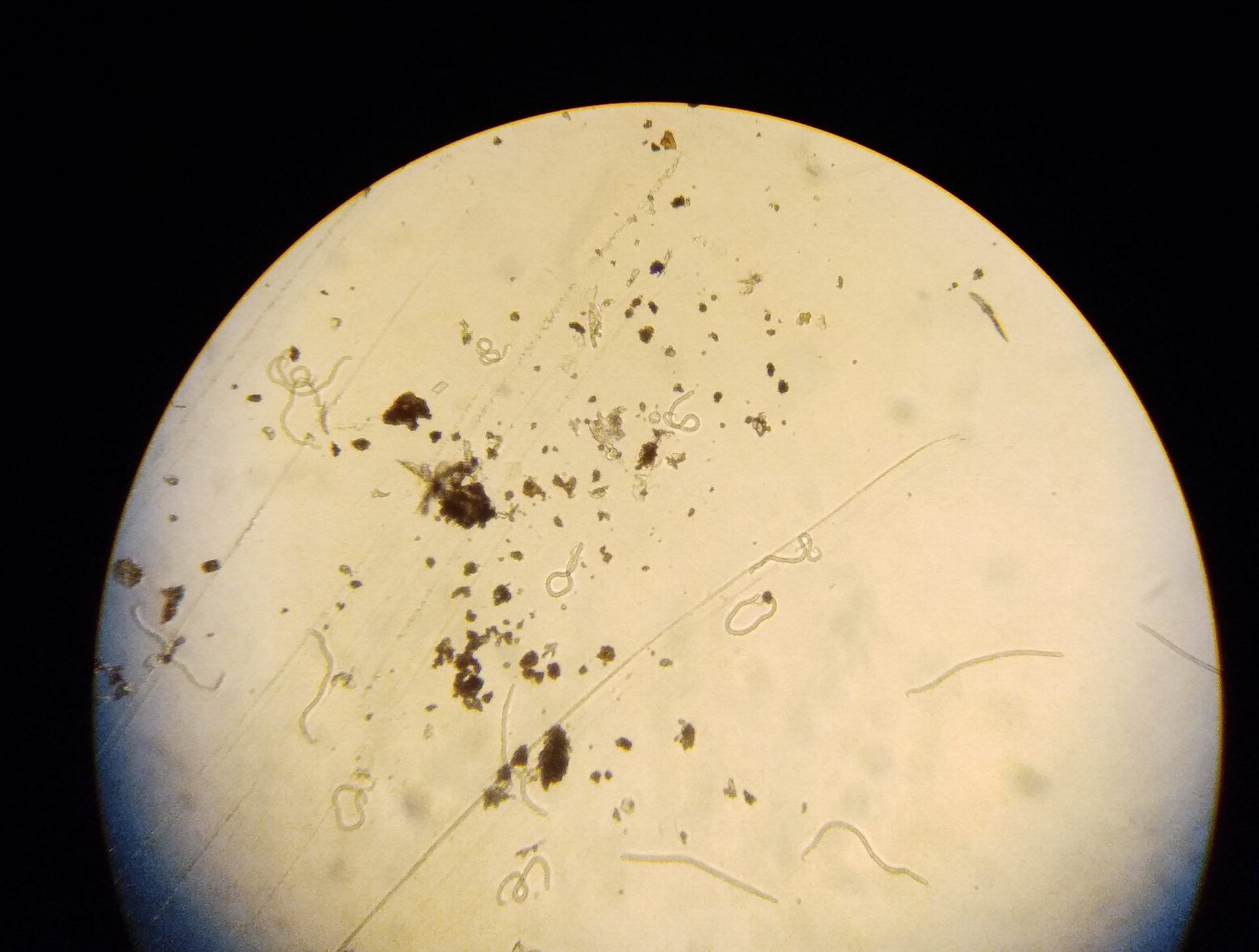
baby worms (microfilariae) of Onchocerca volvulus under the inverted microscope
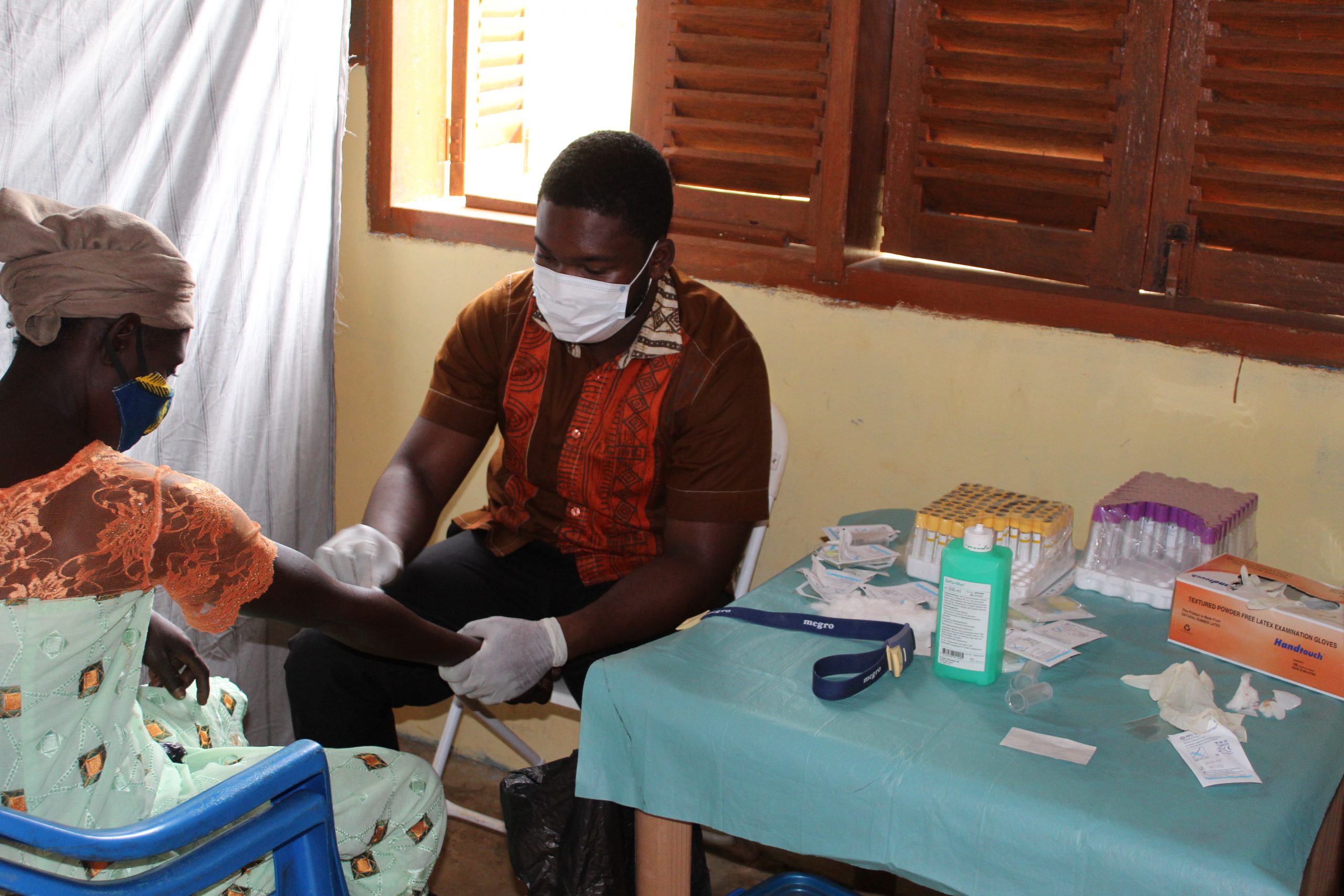
Taking blood samples of study participants on the field for Biochemistry and Haematology analyses and other investigations

Research team members working on the participants’ samples under the Biosafety Cabinet in the field research laboratory

Running the Biochemistry tests on the study participants’ samples to select those who meet the inclusion criteria for the ASTAWOL project.