Work Package 2
Efficacy assessment of Rifampicin and Albendazole to treat lymphatic filariasis patients
There is a global quest to get a drug or combination of drugs, that can be delivered in 10 days or less, with adult worm-killing / macrofilaricidal effects in the treatment of lymphatic filariasis patients. Hence, the need to undertake a clinical trial that aims to shorten the treatment period and assess the safety and adult filarial worm-killing efficacy of the drug combination of rifampicin and albendazole in lymphatic filariasis-infected individuals. It is expected at the end of the trial that, the combination of these two drugs administered continuously for 7 and 14 days, respectively, will be successful in lymphatic filariasis patients. In preclinical studies, the combination of high-dose rifampicin with albendazole for seven days resulted in an increased macrofilaricidal effect. Wolbachia, which is an endosymbiotic bacteria of the lymphatic filariasis filarial worm, was depleted to above a 90% threshold in living adult female worms, predictive of a permanent adult worm-killing activity. Therefore, in this trial, a comparison among enrolled study participants in the following arms will be undertaken:
- Rifampicin plus Albendazole for 7 days
- Rifampicin plus Albendazole for 14 days
- Albendazole only for 14 days and
- Control/placebo / no treatment.
Enrolled study participants in the treatment arms will be treated with a dosage of Rifampicin 35mg/kg/d and Albendazole 400 mg/d. The trial is registered with the Pan African Clinical Trial Registry. For more details on the trial protocol, see PACTR202009704006025.
UPDATE ON WP2
Pre-screening to recruit study participants has been undertaken in lymphatic filariasis-hotspot districts in Ghana, Enrolment and treatment of study participants are yet to be done. During pre-screening, the research procedures were explained to all potential study participants present. Individuals who wanted to participate in the study voluntarily signed or thumb-printed the informed consent forms. Such individuals were then finger-pricked and 75 microlitres (uL) of blood was added to the filarial test strip (FTS) and read following the manufacturer’s instruction. Individuals who were positive for the circulating filarial antigen (CFA) underwent another blood sampling during the night for microfilariae (baby worm of Wuchereria bancrofti) assessment. The pictures below show some of the procedures the research team undertook while carrying out work package 2.

Community members in a queue to be fingerpricked for circulating filarial antigen (CFA) using the filarial test strip (FTS)
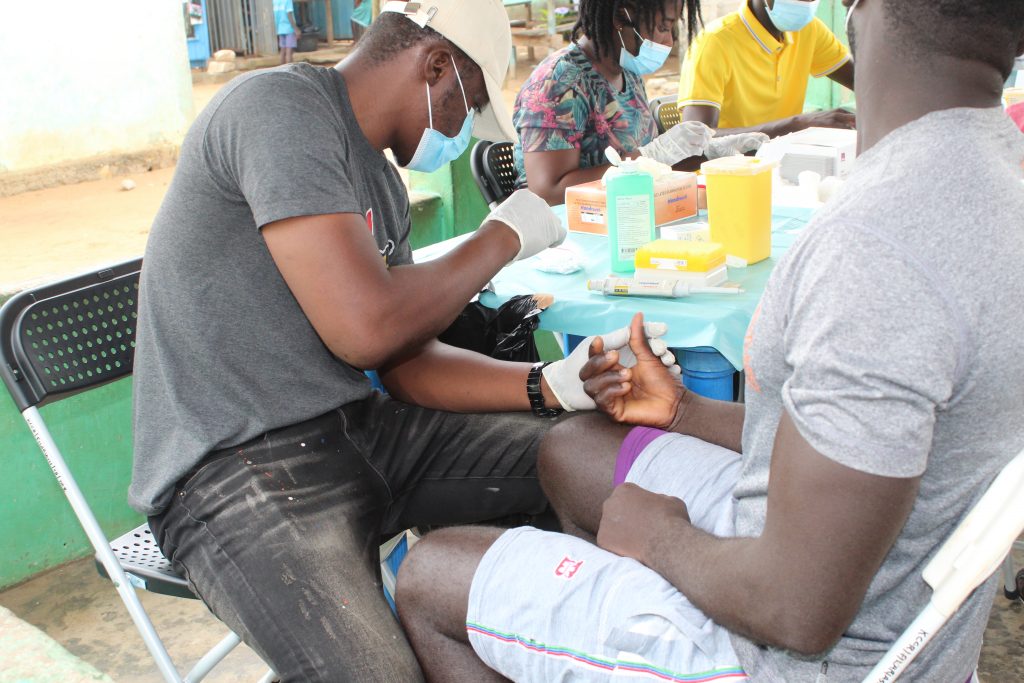
A potential study participant being pricked on the finger to test for the presence of circulating filarial antigen (CFA)
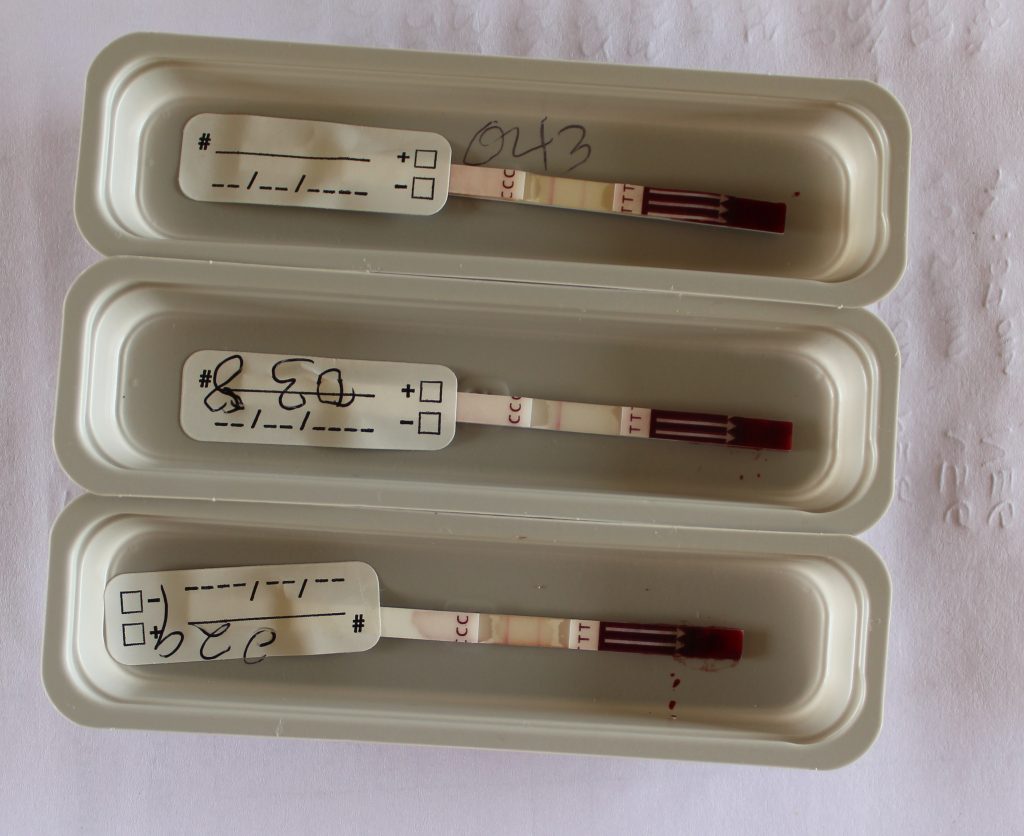
Filarial test strips showing one negative (middle) and two positive (1st and the 3rd) test results